Abstract
Background: A variety of frontline treatment regimens exist for early-stage unfavourable Hodgkin lymphoma (HL), offering personalization of risk versus benefit in this primarily young population of patients. While radiation therapy has been a mainstay of treatment due to improved progression-free survival (PFS), recent studies have challenged this paradigm, using a PET-driven approach (HD17) or incorporating novel agents (nivolumab-AVD (N-AVD), brentuximab-AVD (A-AVD)). Long term risks of radiation and chemotherapy include secondary breast and other cancers, and heart failure; however novel regimens are more costly with uncertainty surrounding long-term efficacy.
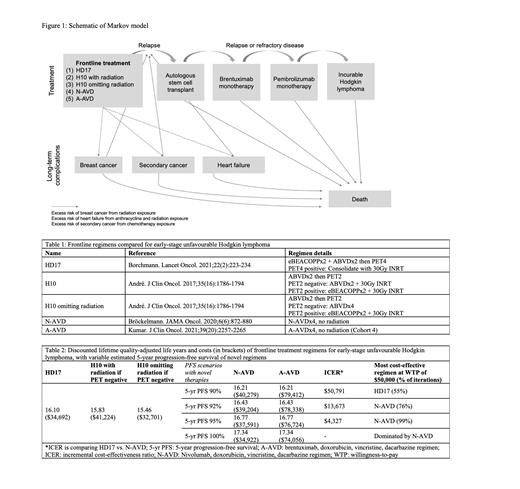
Methods: A cost-effectiveness and cost-utility analysis was conducted to compare five published frontline approaches for early-stage unfavourable HL: HD17, two H10 approaches, N-AVD, and A-AVD without radiation (Table 1). A Markov model was constructed with a lifetime horizon using TreeAge Pro 2021 (Figure 1). The base case was a 20-year-old female with a mediastinal mass who would require chest field radiation. Baseline estimates in the model were derived from the literature, including risk of relapse after each line of therapy, risk of late complications (breast cancer, secondary cancer, and/or heart failure), risk of death (from complications, lymphoma, and background mortality), and health state utilities. A Canadian public health care payer's perspective was taken, and costs are estimated in 2021 Canadian dollars. Global discounting of 3% was used.
Results: Probabilistic sensitivity analyses were performed (10,000 simulations). First, we evaluated the uncertainty of long-term PFS using novel regimens (N-AVD or A-AVD); at a willingness-to-pay of $50,000/QALY, N-AVD was the most cost-effective regimen when 5-year PFS was at least 92% (Table 2). If 5-year PFS with N-AVD was <92%, HD17 became the most cost-effective approach. There was no PFS threshold at which A-AVD was the most cost-effective regimen. Holding the 5-year PFS of novel regimens at 92%, the model remained robust to multiple deterministic sensitivity analyses testing key variables including health state utilities (of relapse post-transplant, breast cancer, second malignancy, heart failure), costs (of radiation, autologous stem cell transplant, breast cancer, second malignancy, heart failure), and risks (of breast cancer after radiation, cardiovascular disease after radiation and/or chemotherapy,). However, if the risk of developing second cancer was less than 2% after 5 years with HD17 approach (current estimates 1% at 48 months in HD17 to 2% at 43 months in HD14 (which used a similar regimen)), or if the median overall survival after secondary cancer was over 9 years, HD17 became the most cost-effective regimen. The threshold cost for brentuximab to make A-AVD the most cost-effective regimen was <$5000 per dose (current price $14,520 CAD).
Conclusions: If the long term PFS of nivolumab-AVD is greater than 92%, it could be the most cost-effective regimen when treating a young female patient with early-stage unfavourable Hodgkin lymphoma. This model accounts for increased costs with nivolumab added to chemotherapy, due to potential reduced incidence of late effects. However, there remain uncertainties in efficacy and risk regarding novel therapies as only non-randomized Phase II studies with short follow-up durations have been published; further trials of these approaches are being planned. HD17 remains the most cost-effective approach among published Phase III regimens. Long term follow-up of HD17 will also be meaningful to understand the risk of second cancer with this approach, which may impact its cost-effectiveness.
Crump: Epizyme: Research Funding; Kyte/Gilead: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Roche: Research Funding. Prica: Astra-Zeneca: Honoraria; Kite Gilead: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal